Some pressure reduction methods¶
| Author: | D.Thaler |
|---|---|
| Created: | Aug 2019 |
| Last changed: | 2019-08-15 |
Reducing the pressure measurment of some place to sea level looks to be an easy task. But several assumptions have to be made about:
- the virtuell stratification of temperature and humidity
- the structure of the gravity field
More or less sophisticated methodes have been developed. Here we simply follow the description of the basic equations and how the recommended methods of WMO and ICAO are related to that.
Basic equations¶
Assuming there is no vertically accelerated windfield the vertical component of the atmospheric equation of motion degenerates to the hydrostatic equation \(\frac{1}{\rho} \frac{\partial p}{\partial z} = -g\). Basic transformations with the help of the ideal gas equation \(\rho = \frac{1}{ R_d} \frac{p}{T}\) gives the general integrated form between the limits \(h_0\) and \(h\):
The outermost right expression is valid with the assumption both \(g\) and \(R_D\) are independent of the geometric height. Because of the fundamental theorem of calculus there is an equivalent formulation:
The barometric mean Temperature \(\overline{T}\) is defined by the integral
All relations above apply to a dry atmosphere. In humid air with variable water vapor content the gas constant should be modified. Instead of working with variable gas constants it’s more practicable to define a temperature with all the necessary changes packed into, the Virtual Temperature:
\(q=\rho_v/\rho\) is the specific humidity, the ratio of the density of water vapor to the density of humid air.
Isothermal Atmosphere¶
Given a vertically constant temperature \(T(z) = \overline{T} = T_0\) the above equation simplifies to:
The inverse function gives the geopotential height as function of the pressure difference for an isothermal atmosphere (a.k.a. hypsometric equation):
that can be used for altimetry.
Politropic Atmosphere¶
A sometimes better approach is the assumption of a linear temperature profile:
Doing the integration yields the solution for pressure as function of the geopotential height:
Solving the above equation for \(h\) gives the hypsometric equation for the polytropic atmosphere:
Density as a function of height can easily be derived by the ideal gas equation \(\rho(h) = p(h)/(R_d T(h))\):
Results¶
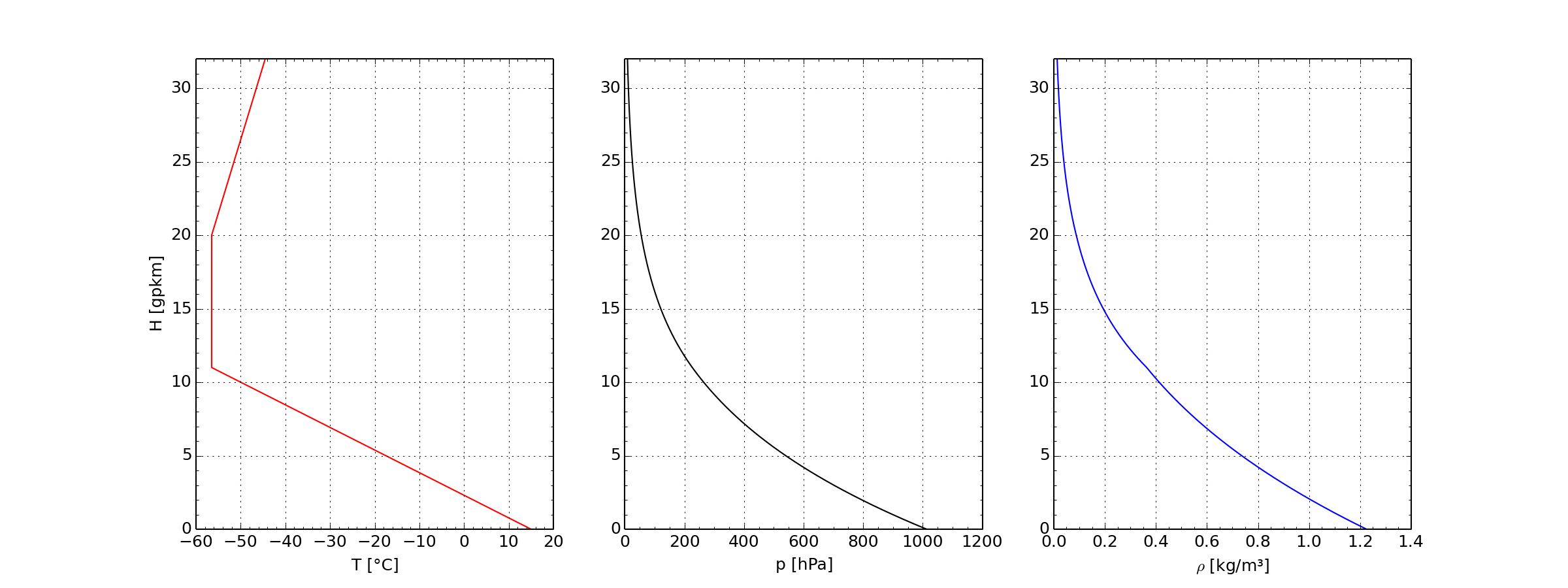
ICAO standard atmosphere up to 32 km height
- Left: Temperature in Celsius, Center: Pressure in Hektopascal,
- Right: Density in Kilogram per Cubic Meter
A table with an appropriate selection of values can be seen here:
| h[gpm] | T[C] | p[hPa] | \(\rho\) [kg/m³] |
|---|---|---|---|
| -300 | 16.95 | 1049.81 | 1.2608 |
| 0 | 15.00 | 1013.25 | 1.2251 |
| 500 | 11.75 | 954.60 | 1.1673 |
| 1000 | 8.50 | 898.74 | 1.1117 |
| 1500 | 5.25 | 845.55 | 1.0581 |
| 2000 | 2.00 | 794.94 | 1.0065 |
| 2500 | -1.25 | 746.81 | 0.9569 |
| 3000 | -4.50 | 701.07 | 0.9092 |
| 4000 | -11.00 | 616.38 | 0.8192 |
| 5000 | -17.50 | 540.18 | 0.7361 |
| 6000 | -24.00 | 471.79 | 0.6597 |
| 7000 | -30.50 | 410.58 | 0.5895 |
| 8000 | -37.00 | 355.97 | 0.5252 |
| 9000 | -43.50 | 307.40 | 0.4663 |
| 10000 | -50.00 | 264.34 | 0.4127 |
| 11000 | -56.50 | 226.30 | 0.3639 |
| 12000 | -56.50 | 193.28 | 0.3108 |
| 14000 | -56.50 | 141.00 | 0.2267 |
| 16000 | -56.50 | 102.86 | 0.1654 |
| 18000 | -56.50 | 75.03 | 0.1207 |
| 20000 | -56.50 | 54.74 | 0.0880 |
| 24000 | -52.50 | 29.30 | 0.0463 |
| 26000 | -50.50 | 21.53 | 0.0337 |
| 28000 | -48.50 | 15.86 | 0.0246 |
| 30000 | -46.50 | 11.72 | 0.0180 |
| 32000 | -44.50 | 8.68 | 0.0132 |
Table of symbols¶
| \(h\) | geopotential height [gpm] |
| \(p\) | pressure [Pa] |
| \(T\) | Temperature [K] |
| \(z\) | geopotential height [gpm] |
| \(\gamma\) | vertical temperature gradient \((-\partial T/\partial z)\) [K/m] |
| \(\rho\) | air density [kg/m³] |
Definitions¶
The ICAO standard atmosphere (ISA) is defined within the meteorological scope as a reference atmosphere for mainly avionic purposes. The atmosphere is considered to be a dry ideal gas. The values of physical constants are shown in the following table:
| Constants for the ICAO standard atmosphere | |
|---|---|
| constant gravity | \(g_0=\) 9.80665 m/s² |
| constant earth radius | \(R=\) 6 356 766 m |
| molar mass of dry air | \(M_d=\) 28.9644 kg/kmol |
| universal gas constant | \(R_*=\) 8 314.32 J/(kmol K) |
| gas constant for dry air | \(R_d=R_*/M_d \approx\) 287.053 J/(kg K) |
ISA starts with surface values of temperature and pressure and continues with different levels of thermal stratification. Levels above 32 km are defined in ISA in 1993 but are neglected here.
| h [km] | T [C] | \(\gamma\) [K/m] | p [hPa] |
|---|---|---|---|
| 0 | +15.0 | 0.0065 | 1013.25 |
| 11 | −56.5 | 0.0000 | |
| 20 | −56.5 | -0.0010 | |
| 32 | −44.5 |
References¶
- Standard meteorological textbook, e.g. Michael Hantel: Einführung Theoretische Metetrologie, Springer-Spektrum, Springer Verlag Berlin-Heidelberg 2013
- ICAO Standard Atmosphäre (Wikipedia-de)
- ICAO Standard Atmosphere (Wikipedia-en)
Any comments to info at foehnwall dot at